The FIONA Study
A 6-month Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy, Safety and PK/PD of an age-and Body Weight-adjusted Oral Finerenone Regimen, in Addition to an ACEI or ARB, for the Treatment of Children, 6 Months to <18 Years of Age, With Chronic Kidney Disease and Proteinuria
What is FIONA?
FIONA is a study sponsored by Bayer AG to learn more about how well the study treatment finerenone works, how safe it is, and how it moves into, through and out of the body, and the effects it has on the body when taken with an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) in children with chronic kidney disease (CKD) and proteinuria. Researchers are looking for a better way to treat children who have chronic kidney disease (CKD), which is long-term kidney disease, and proteinuria, a condition in which a person´s kidneys leak protein into the urine. The main purpose of this study is to learn more about whether finerenone added to either ACEI or ARB can help reduce the amount of protein in the participants’ urine more than a placebo. A placebo looks like a treatment but does not have any medicine in it. Participants will also continue to receive their other medications.
What is CKD, and what is proteinuria?
In children with CKD, the kidney´s filters do not work as well as they should. This can lead to accumulation of waste and fluid in the body and proteinuria, a condition in which a person´s kidneys leak protein into the urine. CKD can lead to other medical problems, such as high blood pressure, also known as hypertension. Vice versa, hypertension and proteinuria can also contribute to worsening of CKD. Therefore, the treatment of CKD aims to control blood pressure and proteinuria.
Why is the study needed?
There are treatments available for doctors to prescribe to children with CKD and hypertension and/or proteinuria. These include “angiotensin-converting enzyme inhibitors” (ACEI) and “angiotensin receptor blockers” (ARB). Both ACEI and ARB can improve kidney function by helping the renin-angiotensin-aldosterone system (RAAS) to work normally. The RAAS is a system that works with the kidneys to control blood pressure and the balance of fluid and electrolytes in the blood. In people with CKD, the RAAS is often too active, which can stop the kidneys from working properly and cause hypertension and proteinuria. However, ACEI or ARB treatment alone might not work for all patients with CKD. The study treatment, finerenone, is expected to potentially help control RAAS overactivation together with an ACEI or ARB. So, the researchers in this study want to learn more about whether finerenone given in addition to either an ACEI or ARB could help their kidney function.
What will the study tell us that is new?
The main purpose of this study is to learn more about whether finerenone added to either ACEI or ARB could help reduce the amount of protein in the participants’ urine more than a placebo.
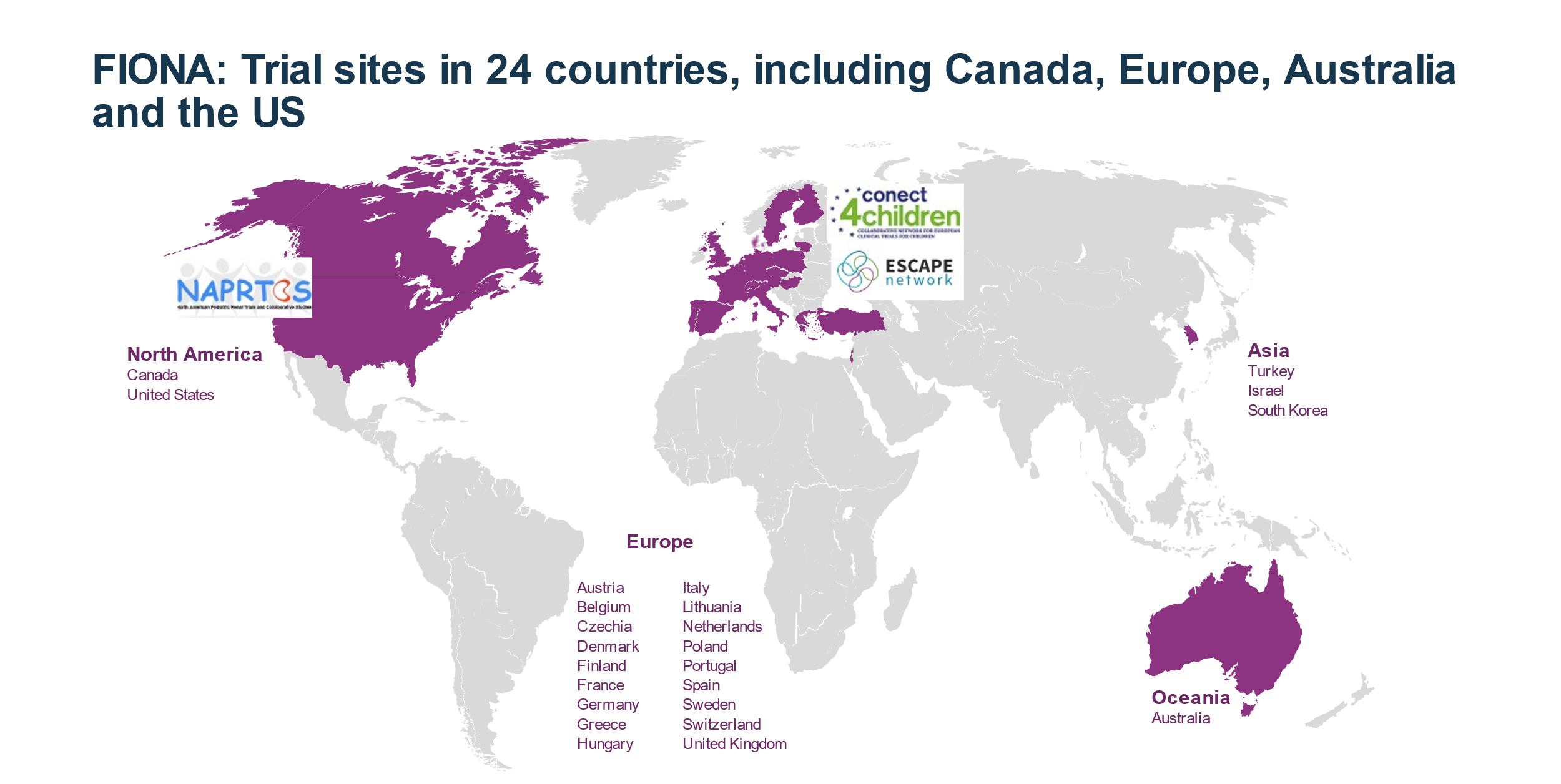
Status and Locations
The FIONA study is currently enrolling patients from 12 to < 18 years of age. More information including a list of current study sites can be found at ClinicalTrials.gov. Please also see also below for an overview of the involved countries.